The revised COVID-19 vaccine, Novavax, has been approved by Health Canada
This protein-based vaccine, named Nuvaxovid, has been reformulated to focus on the JN.1 subvariant of Omicron.
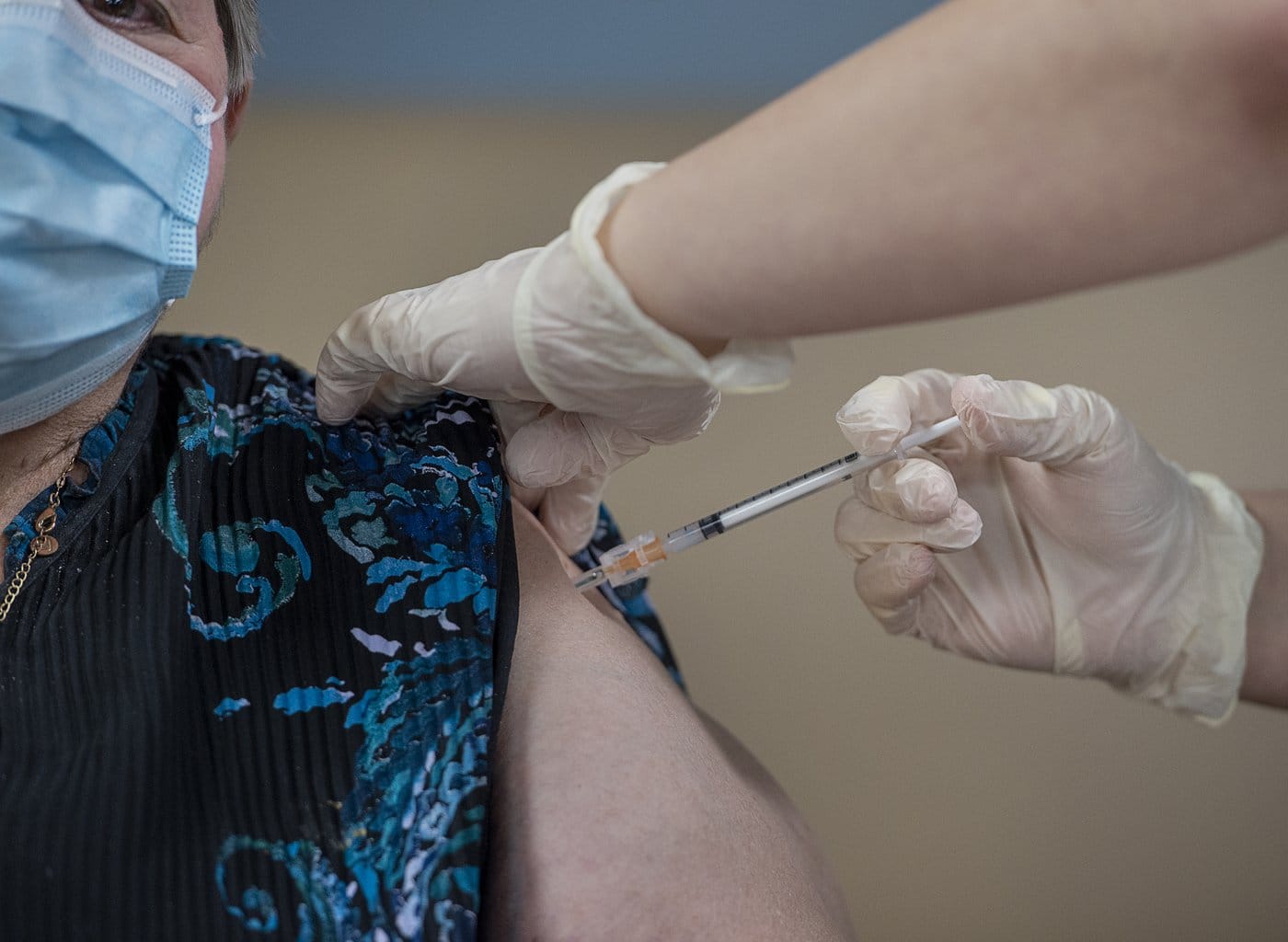
Health Canada has approved Novavax’s updated COVID-19 vaccine, which provides protection against the currently circulating variants of the virus.
This protein-based vaccine, named Nuvaxovid, has been reformulated to focus on the JN.1 subvariant of Omicron.
It will replace the earlier version that targeted the XBB.1.5 subvariant of Omicron.
Recently, Health Canada requested that provinces and territories dispose of their older COVID-19 vaccines to ensure the latest vaccine is utilized during this fall’s respiratory virus season.
Earlier this week, Health Canada also approved Moderna’s updated mRNA COVID vaccine.
A review of Pfizer’s updated mRNA vaccine is still underway, with a decision anticipated soon.